



Orphan Drug and FDA Breakthrough Therapy designations and was approved under the FDA’s Accelerated Approval pathway based on the change from baseline in anti-Factor Xa activity in healthy volunteers. Food and Drug Administration (FDA) has approved Andexxa ®, the first and only antidote indicated for patients treated with rivaroxaban and apixaban, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.Īndexxa received both U.S. ® (Nasdaq:PTLA) today announced that the U.S. SOUTH SAN FRANCISCO, Calif., (GLOBE NEWSWIRE) - Portola Pharmaceuticals, Inc. – Company to Host Conference Call on Friday, at 8:30 a.m. "Andexxa's rapid reversal of the anticoagulating effects of rivaroxaban and apixaban will help clinicians treat life-threatening bleeds, where every minute counts.– Breakthrough Product is a Major Advance in the Treatment of Patients Hospitalized with Life-Threatening Bleeding – "Today's approval represents a significant step forward in patient care and one that the medical community has been eagerly anticipating," ANNEXA-4 chair Stuart J Connolly, MD, McMaster University, Hamilton, Ontario, Canada, said in the release. The median decrease in anti-factor Xa activity from baseline was 90% for rivaroxaban and 93% for apixaban. Among the 185 evaluable high-risk patients in the open-label study, the agent was shown to provide effective clinical hemostasis in 83% of patients out to 12 hours, as recently reported by | Medscape Cardiology.
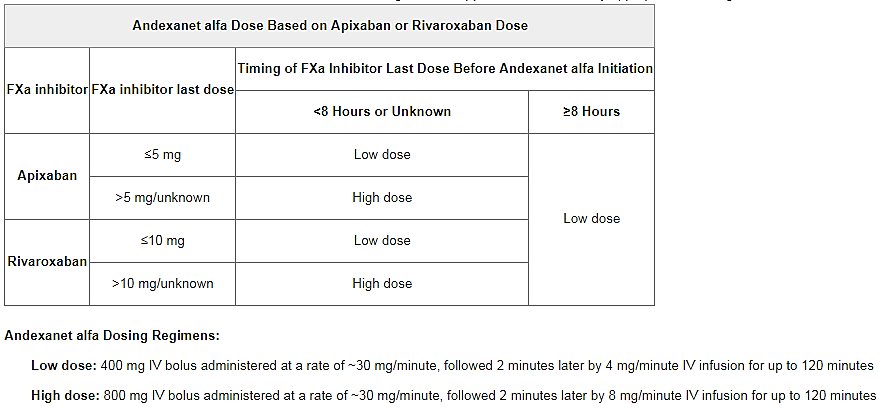
Additional important safety information and full prescribing information are available here.Īpproval of the agent was supported by data from two phase 3 ANNEXA studies, ANNEXA-R and ANNEXA-A, which demonstrated a median decrease in anti-factor Xa activity from baseline of 97% for rivaroxaban and 92% for apixaban, according to a company news release, posted May 3.Īs part of its review, the FDA also assessed interim data from the ongoing ANNEXA-4 study.


 0 kommentar(er)
0 kommentar(er)
